Pipeline
Quoin’s team is relentlessly focused on developing and commercializing innovative treatments for rare and orphan diseases, for which none exists today. Our pipeline comprises three unique products that collectively have the potential to target a broad number of indications, including Netherton Syndrome, Scleroderma, Peeling Skin Syndrome, Ichthyosis, Microcystic Lymphatic Malformations, Angiofibroma, and others. We are committed to continuously expanding our pipeline of products to target additional rare and orphan diseases.
Therapeutic Development Pipeline
| Product Candidate | Indication | Pre-Clinical | Phase 1 | Phase 2 | Phase 3 | Approval | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| QRX003 |
|
|
||||||||||||
| QRX008 |
|
|
||||||||||||
| QRX009 |
|
|
* Pivotal Clinical studies underway
** Clinical trial initiated
*** Clinical testing to commence 1H2026
Quoin Pharmaceuticals launched NETHERTON NOW, a campaign dedicated to raising awareness, fostering advocacy, and supporting patients and families affected by Netherton Syndrome.
VISIT THE WEBSITE: nethertonnow.com
Other Target Indications
Peeling Skin Syndrome
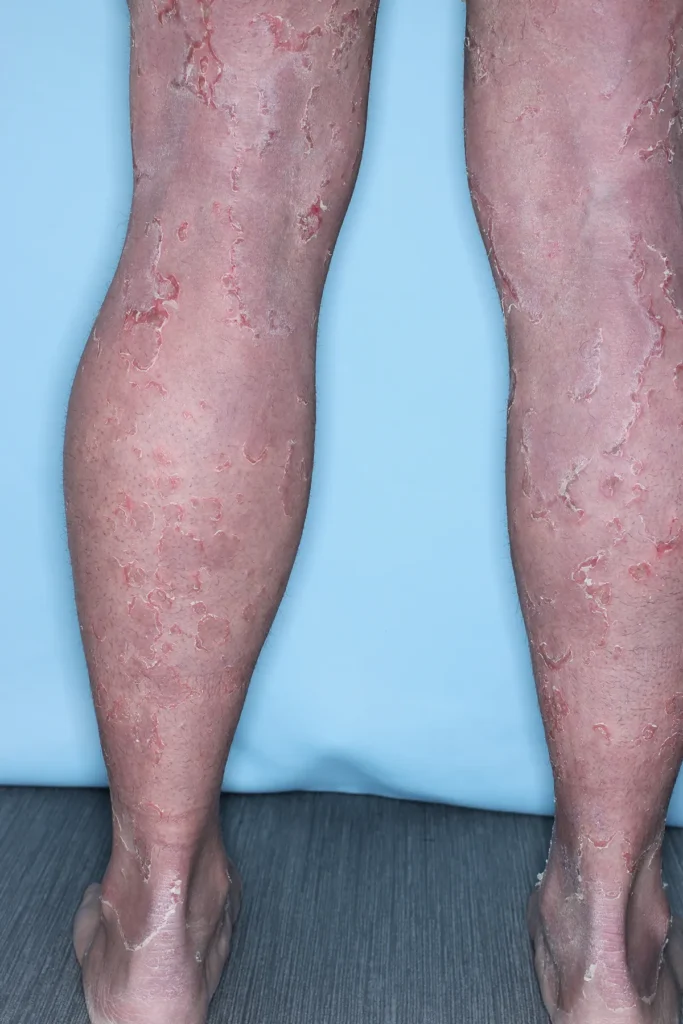
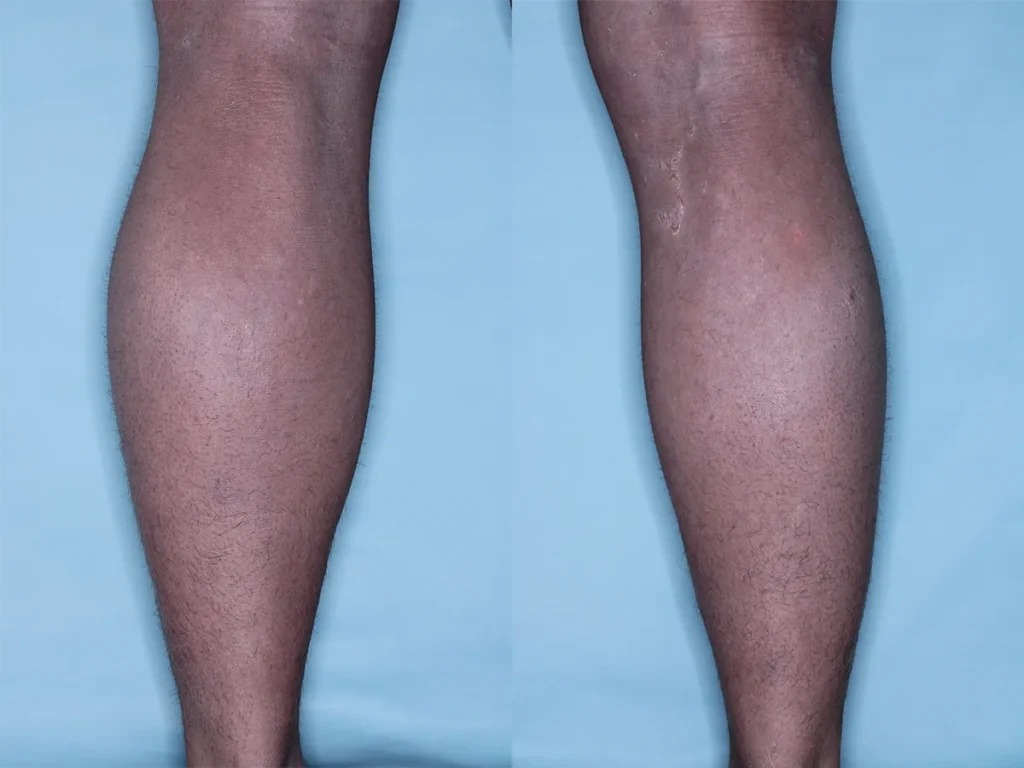
Peeling Skin Syndrome (PSS) is a rare, genetic skin disorder characterized by painless, continual skin peeling due to a separation of the stratum corneum from the underlying layers of the Epidermis. PSS may be generalized, affecting skin over the patient’s entire body, or localized, affecting only the skin of the hands and feet. Generalized PSS can be categorized as non-inflammatory, and less severe, or inflammatory, which is typically more severe and involves other organ systems. There is currently no approved treatment for PSS, and patients manage symptoms using over-the-counter emollients.
Ichthyosis
Ichthyosis refers to a group of more than 30 skin diseases. Typically characterized by dry, scaling, red, itchy skin, the symptoms will vary depending on the type of ichthyosis.
SAM Syndrome
SAM Syndrome, also known as severe skin dermatitis, multiple allergies and metabolic wasting, is a life-threatening rare disease caused by mutations in the desmoglein 1 (DSG1) and desmoplakin (DSP) genes. A compromised skin barrier results in a predisposition to allergies, and other symptoms include severe pruritus, palmoplantar keratoderma, pustular psoriasis and excessive sweating. Quoin is pursuing the development of QRX003 as a potential treatment for SAM Syndrome.
Scleroderma
Scleroderma is a rare, autoimmune disease that affects connective tissue of the skin, blood vessels, internal organs and digestive tract. Symptoms of scleroderma include thickening and tightening of the skin, and inflammation that leads to problems in other organs including the lungs, heart and kidneys. There is currently no approved treatment or cure for scleroderma.

Resources
FIRST Skin Foundation for Ichthyosis and related skin types
Supports patients and families affected by Ichthyosis.
NORD National Organization for Rare Disorders
NORD is leading the fight to improve the lives of patients with rare diseases.
Genetic and Rare Diseases (GARD) Information Center
GARD is a program of the National Institutes of Health (NIH) that provides free access to reliable, easy to understand information about genetic and rare diseases.
Molecular Diagnostic Testing for Netherton Syndrome
Gabriele Richard, MD Associate Professor, Department of Dermatology and Cutaneous Biology and Department of Medicine / Division of Medical Genetics Thomas Jefferson University
Ichthyosis Support Group
Committed to the ongoing provision of an information network and support structure for individuals and families affected by ichthyosis.